Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
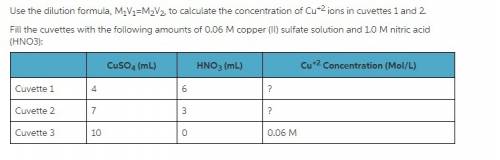
Use the dilution formula, m1v1 = m2v2, to calculate the concentration of cu+2 ions in cuvettes 1 and...
Questions




English, 29.01.2021 20:30

Social Studies, 29.01.2021 20:30




Social Studies, 29.01.2021 20:30

Mathematics, 29.01.2021 20:30


Mathematics, 29.01.2021 20:30

Arts, 29.01.2021 20:30


Mathematics, 29.01.2021 20:30

Computers and Technology, 29.01.2021 20:30



History, 29.01.2021 20:30

History, 29.01.2021 20:30