
Chemistry, 22.07.2019 19:40 Galaxie85111
In a hydrogen molecule, the two hydrogen atoms are held together by a single bond with a bond energy of 436 kj/mol of hydrogen. in other words, to break the h–h bonds in one mole of molecular hydrogen requires the expenditure of 436 kj of energy. using the balanced chemical equation for the formation of water from oxygen and hydrogen (shown above), and interpreting the stoichiometric coefficients as mole amounts, how much energy must be expended in breaking the h–h bonds?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
In a hydrogen molecule, the two hydrogen atoms are held together by a single bond with a bond energy...
Questions

English, 19.05.2020 02:57



Mathematics, 19.05.2020 02:57



Advanced Placement (AP), 19.05.2020 02:57

English, 19.05.2020 02:57

Mathematics, 19.05.2020 02:57

English, 19.05.2020 02:57

Mathematics, 19.05.2020 02:57


Geography, 19.05.2020 02:57







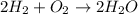

 = 872kJ
= 872kJ

