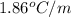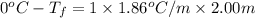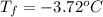Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
Calculate the freezing point of a 2.00 molal solution of the nonelectrolyte glucose. the freezing po...
Questions


Mathematics, 21.05.2020 01:08




Social Studies, 21.05.2020 01:08

Mathematics, 21.05.2020 01:08



Social Studies, 21.05.2020 01:08

Mathematics, 21.05.2020 01:08

Mathematics, 21.05.2020 01:08

History, 21.05.2020 01:08


Advanced Placement (AP), 21.05.2020 01:08



Mathematics, 21.05.2020 01:08


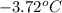
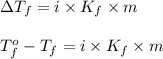
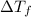 = change in freezing point
= change in freezing point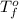 = temperature of pure water =
= temperature of pure water = 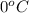
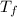 = temperature of solution = ?
= temperature of solution = ?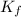 = freezing point constant =
= freezing point constant =