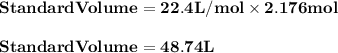Chemistry, 23.07.2019 18:50 loyaltyandgood
Sixty liters of a gas were collected over water when the barometer read 663 mmhg , and the temperature was 20∘c. what volume would the dry gas occupy at standard conditions? (hint: consider dalton's law of partial pressures.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
Sixty liters of a gas were collected over water when the barometer read 663 mmhg , and the temperatu...
Questions

Mathematics, 11.01.2021 18:50

Mathematics, 11.01.2021 18:50

Chemistry, 11.01.2021 18:50

Mathematics, 11.01.2021 18:50


Mathematics, 11.01.2021 18:50






History, 11.01.2021 18:50

Computers and Technology, 11.01.2021 18:50


Mathematics, 11.01.2021 18:50



Business, 11.01.2021 18:50

Mathematics, 11.01.2021 18:50