
Chemistry, 23.07.2019 20:00 bobbyxii6033
Hydrogen sulfate reacts with water to produce sulfate ions and hydronium ions: hso4−+h2o⇌so42−+h3o+ identify the conjugate acid-base pairs.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
Hydrogen sulfate reacts with water to produce sulfate ions and hydronium ions: hso4−+h2o⇌so42−+h3o+...
Questions

Social Studies, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00


English, 05.02.2021 21:00




Mathematics, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00

Social Studies, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00

English, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00

Biology, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00


Chemistry, 05.02.2021 21:00

English, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00


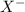 ), and the conjugate acid-base pair is
), and the conjugate acid-base pair is 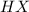 -
- 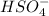 ) is an ion from sulfuric acid. It is still an acid in itself and can "react" with water ((
) is an ion from sulfuric acid. It is still an acid in itself and can "react" with water ((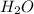 ) to form the sulfate (
) to form the sulfate (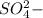 ) and hydronium (
) and hydronium ( )ions.
)ions. 
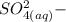 is identified to be the conjugate of the acid
is identified to be the conjugate of the acid 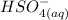 . Thus, the conjugate acid-base pair is
. Thus, the conjugate acid-base pair is 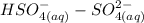 .
.

