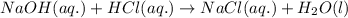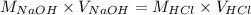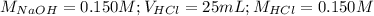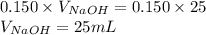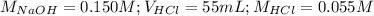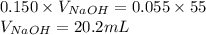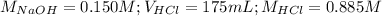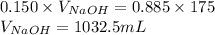Chemistry, 23.07.2019 20:10 mrylenastewart
Determine the volume of 0.150 m naoh solution required to neutralize each sample of hydrochloric acid. the neutralization reaction is: naoh(aq) + hcl(aq) → h2o(l) + nacl(aq) 25 ml of a 0.150 m hcl solution 55 ml of a 0.055 m hcl solution 175 ml of a 0.885 m hcl solution

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
Determine the volume of 0.150 m naoh solution required to neutralize each sample of hydrochloric aci...
Questions

Mathematics, 30.10.2020 20:20

Mathematics, 30.10.2020 20:20



Health, 30.10.2020 20:20

Mathematics, 30.10.2020 20:20

Chemistry, 30.10.2020 20:20



English, 30.10.2020 20:20



Mathematics, 30.10.2020 20:20


Mathematics, 30.10.2020 20:20

Mathematics, 30.10.2020 20:20

Mathematics, 30.10.2020 20:20

Mathematics, 30.10.2020 20:20


Computers and Technology, 30.10.2020 20:20