
Chemistry, 23.07.2019 22:40 anthonyalvar6636
In each pair, identify the solution that will have a higher boiling point. explain. 1. 1.50 moles of lioh (strong electrolyte) and 3.00 moles of koh (strong electrolyte) each in 1.0 kg of water 2. 0.40 mole of al(no3)3 (strong electrolyte) and 0.40 mole of cscl (strong electrolyte) each in 1.0 kg of water

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3


Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
In each pair, identify the solution that will have a higher boiling point. explain. 1. 1.50 moles of...
Questions

Mathematics, 26.01.2020 09:31

Health, 26.01.2020 09:31

Mathematics, 26.01.2020 09:31




History, 26.01.2020 09:31

Mathematics, 26.01.2020 09:31


Mathematics, 26.01.2020 09:31


Chemistry, 26.01.2020 09:31

Geography, 26.01.2020 09:31


History, 26.01.2020 09:31

Mathematics, 26.01.2020 09:31



Mathematics, 26.01.2020 09:31

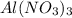 in 1.0 kg of water.
in 1.0 kg of water.
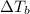 is the elevation in boiling point, i is the Van't hoff factor, m is the molality of the solution and
is the elevation in boiling point, i is the Van't hoff factor, m is the molality of the solution and 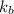 is the molal elevation constant for the solvent used.
is the molal elevation constant for the solvent used.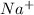 and
and 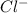 . So, the value of i for NaCl is 2.
. So, the value of i for NaCl is 2. 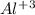 ion and three
ion and three 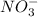 ions. So, the value of i for this is 4.
ions. So, the value of i for this is 4. 


