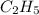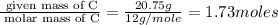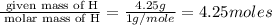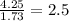Chemistry, 24.07.2019 09:00 whiteshawn02
Asample of a hydrocarbon contains 20.75 g c and 4.25 g h. its molar mass is 58.04 g/mol. what is its empirical formula c2h5 ch2 ch c5h

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
Asample of a hydrocarbon contains 20.75 g c and 4.25 g h. its molar mass is 58.04 g/mol. what is its...
Questions

English, 30.03.2020 23:02



Mathematics, 30.03.2020 23:02


Mathematics, 30.03.2020 23:02





Mathematics, 30.03.2020 23:02



English, 30.03.2020 23:03

Mathematics, 30.03.2020 23:03



Mathematics, 30.03.2020 23:03

Chemistry, 30.03.2020 23:03

Mathematics, 30.03.2020 23:03