
Chemistry, 25.07.2019 02:00 JocelynC7237
Suppose you titrate 80.0 ml of 2.00 m naoh with 20.0 ml of 4.00 m hcl. what is the final concentration of oh− ions.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
4nh3+5o2--> 4no+6h20what is the total number of moles of h2o produced when 12 mole of nh3 is completely consumed?
Answers: 3

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1
You know the right answer?
Suppose you titrate 80.0 ml of 2.00 m naoh with 20.0 ml of 4.00 m hcl. what is the final concentrati...
Questions


Mathematics, 25.06.2019 15:00

Social Studies, 25.06.2019 15:00

Mathematics, 25.06.2019 15:00



Computers and Technology, 25.06.2019 15:00


Mathematics, 25.06.2019 15:00

Mathematics, 25.06.2019 15:00

Mathematics, 25.06.2019 15:00







History, 25.06.2019 15:00

Mathematics, 25.06.2019 15:00

Mathematics, 25.06.2019 15:00

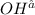 is 0.8 M.
is 0.8 M.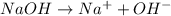
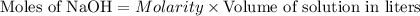
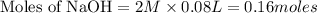
![[OH^-]](/tpl/images/0129/3993/b2910.png)


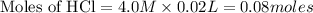
![[H^+]](/tpl/images/0129/3993/07acb.png)

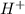 will react with 0.08 moles of
will react with 0.08 moles of 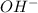 and (0.16-0.08)= 0.08 moles of
and (0.16-0.08)= 0.08 moles of ![[OH^]-=\frac{moles}{\text {Volume in L}}=\frac{0.08}{0.1}=0.8M](/tpl/images/0129/3993/83da4.png)


