
Chemistry, 25.07.2019 09:00 dathanboyd
Which of the following does not show the law of conservation of mass? \ a. 24 g of mg burn in 32 g o2 to produce 56 g of mgo. b. 18 g of mg burn in 24 g o2 to produce 24 g of mgo. c. 2 atoms of mg react with 1 molecule of o2 to produce 2 units of mgo. d. 1 atom of mg reacts with 1 atom of o to produce a unit of mgo that contains 2 atoms.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3


Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2
You know the right answer?
Which of the following does not show the law of conservation of mass? \ a. 24 g of mg burn in 32 g...
Questions

History, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01




English, 20.09.2020 04:01

Biology, 20.09.2020 04:01


English, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01



Mathematics, 20.09.2020 04:01

Geography, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01



Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

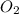 to produce 56 g of MgO. So, mass of reactants is (24 g + 32 g) = 56 g. Mass of products is 56 g. Hence, it is following law of conservation of mass.
to produce 56 g of MgO. So, mass of reactants is (24 g + 32 g) = 56 g. Mass of products is 56 g. Hence, it is following law of conservation of mass.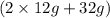 = 56 g. Mass of products is
= 56 g. Mass of products is 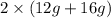 = 56 g. Hence, it is following law of conservation of mass.
= 56 g. Hence, it is following law of conservation of mass.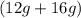 = 28 g. Mass of products is (12 g + 16 g) = 28 g. Hence, it is following law of conservation of mass.
= 28 g. Mass of products is (12 g + 16 g) = 28 g. Hence, it is following law of conservation of mass.

