
Chemistry, 25.07.2019 11:20 graceaowen
The pressure in a 12.2 l vessel that contains 2.34 g of carbon dioxide, 1.73 g of sulfur dioxide, and 3.33 g of argon, all at 42°c is torr.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
The pressure in a 12.2 l vessel that contains 2.34 g of carbon dioxide, 1.73 g of sulfur dioxide, an...
Questions

Mathematics, 02.07.2019 05:00

Social Studies, 02.07.2019 05:00

Mathematics, 02.07.2019 05:00


Mathematics, 02.07.2019 05:00


Mathematics, 02.07.2019 05:00


English, 02.07.2019 05:00

Social Studies, 02.07.2019 05:00

Mathematics, 02.07.2019 05:00

Biology, 02.07.2019 05:00

Mathematics, 02.07.2019 05:00


Mathematics, 02.07.2019 05:00


Mathematics, 02.07.2019 05:00

Biology, 02.07.2019 05:00


Mathematics, 02.07.2019 05:00

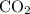 , 1.73 g of
, 1.73 g of 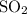 and 3.33 g of Ar is
and 3.33 g of Ar is 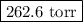 .
.
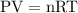 …… (1)
…… (1)
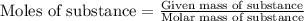 ...... (2)
...... (2)
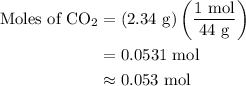
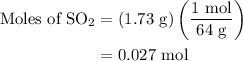
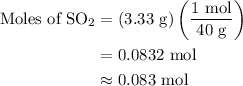
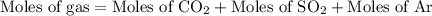 ...... (3)
...... (3)
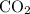 , 0.027 mol for the moles of
, 0.027 mol for the moles of 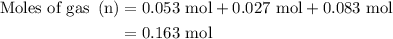
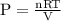 ...... (4)
...... (4)
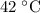 for T and
for T and 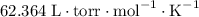 for R in equation (4).
for R in equation (4).



