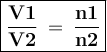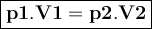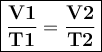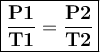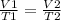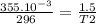Chemistry, 25.07.2019 12:40 joseflores10205
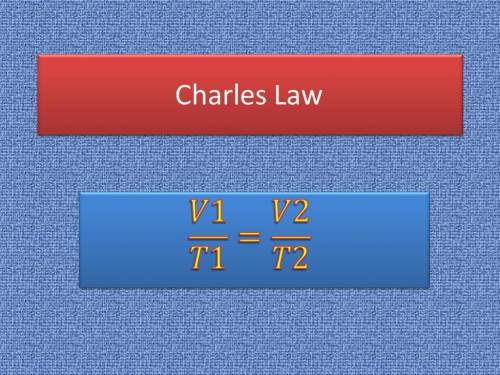
Asample of helium gas occupies 355ml at 23°c. if the container the he is in is expanded to 1.50 l at constant pressure, what is the final temperature for the he at this new volume?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 23.06.2019 10:10
In a covalent bond, two atoms are held together by the attraction between . the number of covalent bonds that an atom can form depends on the number of in the atom.
Answers: 2

Chemistry, 23.06.2019 11:30
Bridget is in science class. her teacher gives her two unknown substances and asks her to determine their relative ph. she places a piece of red litmus paper into both substances. the litmus paper turns purple when she places it into substance i. the litmus paper turns blue when she places it into substance ii. a. substance i is a neutral substance and substance ii is an acid. b. substance i is a neutral substance and substance ii is a base. c. substance i is an acid and substance ii is a base. d. substance i is a base and substance ii is a neutral substance.
Answers: 1

Chemistry, 23.06.2019 16:00
Which of the following is a reason to make an armature the parent of a creature
Answers: 1
You know the right answer?
Asample of helium gas occupies 355ml at 23°c. if the container the he is in is expanded to 1.50 l at...
Questions

Mathematics, 10.07.2019 23:30


English, 10.07.2019 23:30




Mathematics, 10.07.2019 23:30

Chemistry, 10.07.2019 23:30


Biology, 10.07.2019 23:30



History, 10.07.2019 23:30





Mathematics, 10.07.2019 23:30


Social Studies, 10.07.2019 23:30