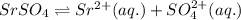Chemistry, 26.07.2019 16:20 brandydailey24pe8r24
Strontium sulfate becomes less soluble in an aqueous solution when sodium sulfate is added because? 1)the addition of sulfate ions shifts equilibrium to the left. 2)the addition of sodium ions shifts equilibrium to the left. 3)the addition of sulfate ions shifts equilibrium to the right. 4)the addition of sodium ions shifts equilibrium to the right.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3
You know the right answer?
Strontium sulfate becomes less soluble in an aqueous solution when sodium sulfate is added because?...
Questions








Social Studies, 07.03.2020 05:08




Mathematics, 07.03.2020 05:08