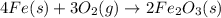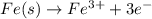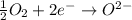Chemistry, 27.07.2019 11:10 graymonky12
Why is rusted iron an example of an oxidation–reduction reaction? electrons are exchanged?

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1
You know the right answer?
Why is rusted iron an example of an oxidation–reduction reaction? electrons are exchanged?...
Questions

Computers and Technology, 02.12.2021 19:10

Computers and Technology, 02.12.2021 19:10



Mathematics, 02.12.2021 19:10

Mathematics, 02.12.2021 19:10


Social Studies, 02.12.2021 19:10

Spanish, 02.12.2021 19:10



History, 02.12.2021 19:10

Biology, 02.12.2021 19:10

History, 02.12.2021 19:10

Mathematics, 02.12.2021 19:10


Mathematics, 02.12.2021 19:10

Chemistry, 02.12.2021 19:10

English, 02.12.2021 19:10

Chemistry, 02.12.2021 19:10