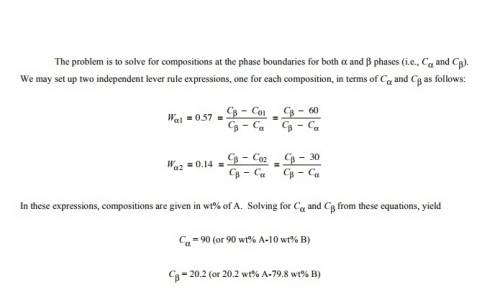
For alloys of two hypothetical metals a and b, there exist an α, a-rich phase and a β, b-rich phase. from the mass fractions of both phases for two different alloys (given below), which are at the same temperature, determine the composition of the phase boundary (or solubility limit) for (a)α and (b)β phases at this temperature.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
For alloys of two hypothetical metals a and b, there exist an α, a-rich phase and a β, b-rich phase....
Questions


Mathematics, 25.05.2020 10:58

Mathematics, 25.05.2020 10:58




English, 25.05.2020 10:58

Health, 25.05.2020 10:58




Health, 25.05.2020 11:57



Mathematics, 25.05.2020 11:57


Geography, 25.05.2020 11:57


Mathematics, 25.05.2020 11:57

History, 25.05.2020 11:57