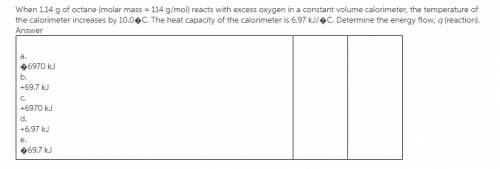
Chemistry, 27.07.2019 18:00 mooreadrian412
When 1.14 g of octane (molar mass = 114 g/mol) reacts with excess oxygen in a constant volume calorimeter, the temperature of the calorimeter increases by 10.0 c?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:10
Amonoprotic acid is an acid that donates a single proton to the solution. suppose you have 0.140 g of a monoprotic acid dissolved in 35.0 ml of water. this solution is then neutralized with 14.5 ml of 0.110 m naoh. what is the molar mass of the acid?
Answers: 1



You know the right answer?
When 1.14 g of octane (molar mass = 114 g/mol) reacts with excess oxygen in a constant volume calori...
Questions

Biology, 05.01.2020 19:31


History, 05.01.2020 19:31

Mathematics, 05.01.2020 19:31

Mathematics, 05.01.2020 19:31

English, 05.01.2020 19:31

Mathematics, 05.01.2020 19:31


History, 05.01.2020 19:31


Biology, 05.01.2020 19:31

Mathematics, 05.01.2020 19:31







Health, 05.01.2020 20:31