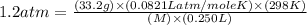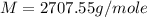Chemistry, 27.07.2019 18:40 krazziekidd2p845ri
An aqueous solution of a soluble compound (a nonelectrolyte) is prepared by dissolving 33.2 g of the compound in sufficient water to form 250 ml of solution. the solution has an osmotic pressure of 1.2 atm at 25 °c. what is the molar mass (g/mole) of the compound?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
An aqueous solution of a soluble compound (a nonelectrolyte) is prepared by dissolving 33.2 g of the...
Questions







History, 21.04.2020 19:36


English, 21.04.2020 19:36

Mathematics, 21.04.2020 19:37






Biology, 21.04.2020 19:37

Chemistry, 21.04.2020 19:37



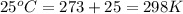
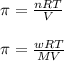
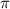 = osmotic pressure
= osmotic pressure