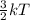Chemistry, 28.07.2019 04:00 carolyntowerskemp
Molecules that have longer arrows are moving faster. which statement describes what is happening in this system? a) molecules vibrate more as temperature decreases. b)temperature is not related to the average kinetic energy of a system. c)kinetic energy decreases as temperature decreases. d)there is no change in average kinetic energy between these two systems.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

You know the right answer?
Molecules that have longer arrows are moving faster. which statement describes what is happening in...
Questions

Biology, 21.01.2020 23:31

Mathematics, 21.01.2020 23:31

Chemistry, 21.01.2020 23:31



Mathematics, 21.01.2020 23:31




Mathematics, 21.01.2020 23:31



Physics, 21.01.2020 23:31




History, 21.01.2020 23:31