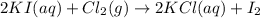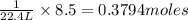Answers: 1
Another question on Chemistry

Chemistry, 20.06.2019 18:04
Brad and his family like to go camping. they always take a gas lantern with them. the lantern can take fiuels but always puts out the same amount of light. brad has two different kinds of fuel for the lantern. how can he figure out which kind of fuel has more chemical energy that the lantern can turn into light? measure the brightness of the lantern using each kind of fuel by comparing it to the full moon. g measure the volume of the containers of fuel. measure how long the lantern stays lit on a fixed amount of fuel. measure the mass of each container of fuel using a balance scale. answer 12
Answers: 1

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2
You know the right answer?
Assume that 8.5 l of iodine gas (i2) are produced at stp according to the following balanced equatio...
Questions











Social Studies, 24.03.2020 20:48



Mathematics, 24.03.2020 20:48



Biology, 24.03.2020 20:48



History, 24.03.2020 20:48