
Amolecule, that is sp3d2 hybridized and has a molecular geometry of square pyramidal, has bonding groups and lone pairs around its central atom a molecule, that is hybridized and has a molecular geometry of square pyramidal, has bonding groups and lone pairs around its central atom 5, 1 4, 2 4, 1 3, 2 2, 3

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Amolecule, that is sp3d2 hybridized and has a molecular geometry of square pyramidal, has bonding g...
Questions








History, 04.07.2019 04:30


Computers and Technology, 04.07.2019 04:30


Mathematics, 04.07.2019 04:30

History, 04.07.2019 04:30




History, 04.07.2019 04:30

Mathematics, 04.07.2019 04:30

Social Studies, 04.07.2019 04:30

![\text{Number of electron pairs} =\frac{1}{2}[V+N-C+A]](/tpl/images/0144/4003/cb4fc.png)
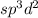 , this means that the number of electron pairs are 6. The molecular geometry of the molecule having electron pairs as 6 is octahederal.
, this means that the number of electron pairs are 6. The molecular geometry of the molecule having electron pairs as 6 is octahederal.

