
Chemistry, 29.07.2019 19:00 elitehairnerd1964
Which of the following best explains the polarity of water (h2o)? a. protons are more attracted to the hydrogen atoms, so the hydrogen atoms become positively charged. b. since the oxygen atom has more electrons than the hydrogen atoms, it is negatively charged. c. electrons are more attracted to the oxygen atom, so there is a partial negative charge on the oxygen atom. d. since there are two positive hydrogen atoms and only one negative oxygen atom, the water molecule is positively charged.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Which of the following best explains the polarity of water (h2o)? a. protons are more a...
Questions

Mathematics, 06.11.2020 19:30

Mathematics, 06.11.2020 19:30

English, 06.11.2020 19:30











English, 06.11.2020 19:30


History, 06.11.2020 19:30



Biology, 06.11.2020 19:30

Mathematics, 06.11.2020 19:30

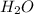 . This shows that there are two hydrogen atoms and one oxygen atom.
. This shows that there are two hydrogen atoms and one oxygen atom. 

