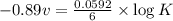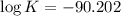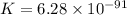Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
What is the value of the equilibrium constant for this redox reaction? 2cr3+(aq) + 3sn2+ (aq) 2cr(s...
Questions

Mathematics, 16.04.2021 05:10

English, 16.04.2021 05:10

Spanish, 16.04.2021 05:10

Mathematics, 16.04.2021 05:10



Mathematics, 16.04.2021 05:10











History, 16.04.2021 05:10


Mathematics, 16.04.2021 05:10

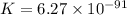
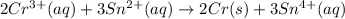
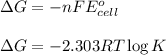
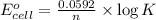 ........(1)
........(1)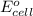 = cell potential = -0.89 v
= cell potential = -0.89 v