Can someone check my anser plase
protons and electrons naturally repel each other; the...

Chemistry, 07.11.2019 20:31 ballislifeqw3089
Can someone check my anser plase
protons and electrons naturally repel each other; therefore, protons stay in the nucleus.
true
false
protons and electrons naturally attract each other, but the nucleus overcomes this electrostatic interaction by nuclear forces.
false
the two forces most responsible for holding the atom together are:
gravitational and weak
electromagnetic and strong nuclear
strong nuclear and weak
gravitational and electromagnetic
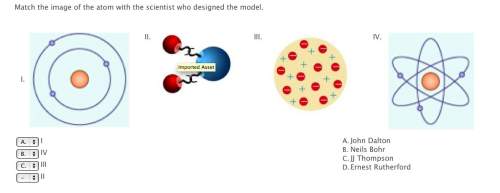
rutherford's model of the atom was similar to bohr's model because they both thought that:
electrons were located in the nucleus of the atom
the nucleus of the atom was centrally located in the atom and was positively
the nucleus was negatively charged and that the electrons were positively charged
electrons were too small in size to affect the properties of atoms
bohr looked to improve upon rutherford's model of the atom because bohr thought that rutherford's model:
was totally incorrect
needed to relocate the protons and neutrons
was not stable because the electrons did not have set
did not account for the positive charge of the protons
one of the important shortcomings of the bohr model of the atom was that:
it did not distinguish between protons and electrons
it specified both definite location and momentum (energy) for an
it showed that all electrons have equal energies
electron energies cannot be calculated
the scientist most often credited with the idea that a quantum of light (photon) can act as a particle is:
schrödinger
de broglie
heisenberg
the heisenberg uncertainty principle states that:
the wave nature and the particle nature of matter are unrelated to one another
the wave nature and the particle nature of matter are complementary
the wave nature and the particle nature of matter are directly proportional to one another
the wave nature and the particle nature of matter are both able to be precisely calculated
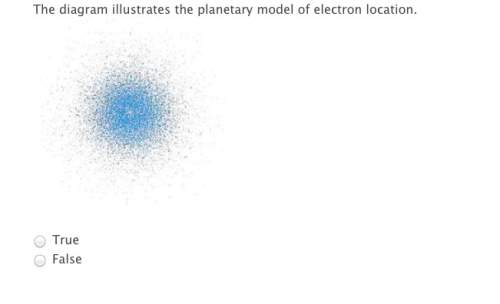
electrons occupy certain high probability areas known as orbitals.
false
i need on the images also


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
Questions

Mathematics, 14.12.2020 09:30


Biology, 14.12.2020 09:30


World Languages, 14.12.2020 09:30


Mathematics, 14.12.2020 09:30

Mathematics, 14.12.2020 09:30






Mathematics, 14.12.2020 09:30


Advanced Placement (AP), 14.12.2020 09:30




Mathematics, 14.12.2020 09:30



