Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

You know the right answer?
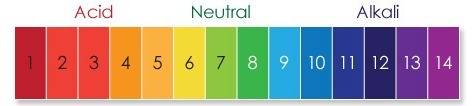
Fish give off the compound ammonia which has a ph above 7 to which class of compounds does ammonia b...
Questions

Social Studies, 28.09.2019 20:00

Social Studies, 28.09.2019 20:00

Health, 28.09.2019 20:00



Mathematics, 28.09.2019 20:00

Biology, 28.09.2019 20:00



History, 28.09.2019 20:00


Health, 28.09.2019 20:00

Mathematics, 28.09.2019 20:00

Chemistry, 28.09.2019 20:00

Chemistry, 28.09.2019 20:00

English, 28.09.2019 20:00

Mathematics, 28.09.2019 20:00

History, 28.09.2019 20:00

Mathematics, 28.09.2019 20:00

History, 28.09.2019 20:00