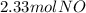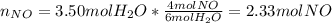`suppose you were tasked with producing some nitrogen monoxide (a. k.a. nitric oxide). i'm sure this is often requested of you. you can do it by combusting ammonia (be the equation would be as follows: 4nh3 + 5o 2→ 4no + 6h2o. if you form 3.50 mol of water, how much no forms? a. 2.33 mol. b. 4.00 mol. c. 4.50 mol. d. 6.75 mol. e. none of the above

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3



Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
`suppose you were tasked with producing some nitrogen monoxide (a. k.a. nitric oxide). i'm sure this...
Questions


Mathematics, 07.04.2020 16:05

Mathematics, 07.04.2020 16:05

Mathematics, 07.04.2020 16:05






Mathematics, 07.04.2020 16:06


Mathematics, 07.04.2020 16:06





Mathematics, 07.04.2020 16:07