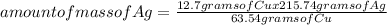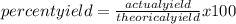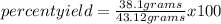Chemistry, 31.07.2019 08:50 gbrbogdan9665
In a particular reaction between copper metal and silver nitrate, 12.7 g cu produced 38.1 g ag. what is the percent yield of silver in this reaction? cu + 2agno3 → cu(no3)2 + 2ag

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
In a particular reaction between copper metal and silver nitrate, 12.7 g cu produced 38.1 g ag. what...
Questions


Mathematics, 18.03.2021 08:50



English, 18.03.2021 08:50


History, 18.03.2021 08:50





Biology, 18.03.2021 08:50





Mathematics, 18.03.2021 08:50

History, 18.03.2021 08:50

Mathematics, 18.03.2021 08:50