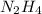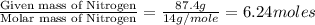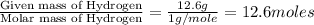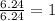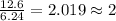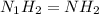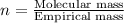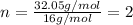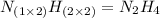Chemistry, 31.07.2019 15:10 jphelps19992019
An analysis of a compound showed it contained 87.4% nitrogen and 12.6% hydrogen. the molecular weight was determined to be 32.05 g/mol. what is the molecular formula for this compound?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
An analysis of a compound showed it contained 87.4% nitrogen and 12.6% hydrogen. the molecular weigh...
Questions






Mathematics, 14.04.2021 01:30

Mathematics, 14.04.2021 01:30

Mathematics, 14.04.2021 01:30



History, 14.04.2021 01:30




Chemistry, 14.04.2021 01:30

English, 14.04.2021 01:30


History, 14.04.2021 01:30

Mathematics, 14.04.2021 01:30

English, 14.04.2021 01:30