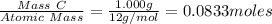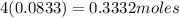Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1


Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2
You know the right answer?
Carbon tetrachloride contains one carbon and four chlorine atoms. for this compound 11.818 g of chlo...
Questions



Physics, 03.07.2019 20:00

English, 03.07.2019 20:00


Arts, 03.07.2019 20:00

Mathematics, 03.07.2019 20:00

English, 03.07.2019 20:00


Mathematics, 03.07.2019 20:00






Mathematics, 03.07.2019 20:00


Social Studies, 03.07.2019 20:00


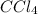 contains one carbon (C) and for chlorine (Cl) atoms
contains one carbon (C) and for chlorine (Cl) atoms