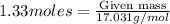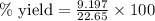Chemistry, 01.08.2019 21:30 05leslun42715
Ammonia (nh3) can be produced by the reaction of hydrogen gas with nitrogen gas: 3h2 + n2 = 2nh3 a chemist reacts 2.00 mol h2 with excess n2. the reaction yields 0.54 mol nh3. what is the percent yield of the reaction? a) 25% b) 40% c) 60% d) 80%

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

You know the right answer?
Ammonia (nh3) can be produced by the reaction of hydrogen gas with nitrogen gas: 3h2 + n2 = 2nh3 a...
Questions

Mathematics, 11.11.2021 14:00

German, 11.11.2021 14:00

Chemistry, 11.11.2021 14:00


English, 11.11.2021 14:00


History, 11.11.2021 14:00


Mathematics, 11.11.2021 14:00

Mathematics, 11.11.2021 14:00

Mathematics, 11.11.2021 14:00

Mathematics, 11.11.2021 14:00

Spanish, 11.11.2021 14:00


Social Studies, 11.11.2021 14:00





Mathematics, 11.11.2021 14:00

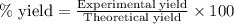 ....(1)
....(1)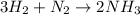
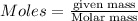 ....(2)
....(2)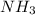 = 17.031g/mol
= 17.031g/mol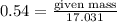
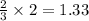 moles of ammonia.
moles of ammonia.