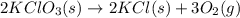Chemistry, 28.09.2019 14:10 mckadams02
Potassium chlorate (kclo3) decomposes in a reaction described by this chemical equation: 2kclo3(s) → 2kcl(s) + 3o2(g) if 36.0 grams of potassium chlorate enter into this reaction, what is the total mass of the two products? 90.0 grams 54.0 grams 18.0 grams 36.0 grams

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1


Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
Potassium chlorate (kclo3) decomposes in a reaction described by this chemical equation: 2kclo3(s)...
Questions

Mathematics, 27.05.2021 01:00



Social Studies, 27.05.2021 01:00


Business, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00


Mathematics, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00

Mathematics, 27.05.2021 01:00

English, 27.05.2021 01:00

History, 27.05.2021 01:00



Mathematics, 27.05.2021 01:00