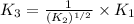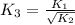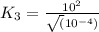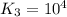Chemistry, 02.08.2019 06:40 kenldykido2300
For the hypothetical reactions 1 and 2, k1 = 102 and k2 = 10–4. 1. a2(g) + b2(g) 2ab(g) 2. 2a2(g) + c2(g) 2a2c(g) 3. a2c(g) + b2(g) 2ab(g) + (1/2)c2(g) what is the value for k for reaction 3?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
For the hypothetical reactions 1 and 2, k1 = 102 and k2 = 10–4. 1. a2(g) + b2(g) 2ab(g) 2. 2a2(g) +...
Questions




Mathematics, 13.12.2019 03:31





Chemistry, 13.12.2019 03:31

Arts, 13.12.2019 03:31




Mathematics, 13.12.2019 03:31



English, 13.12.2019 03:31

Physics, 13.12.2019 03:31


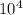
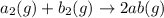 ;
; 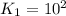
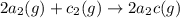 ;
; 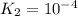
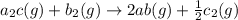
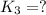
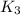 for the final reaction.
for the final reaction.