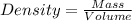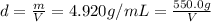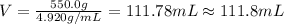Chemistry, 04.08.2019 00:00 andershy1405
Asample of an unknown metal (density = 4.920 g/ml) weighs 550.0 g. what is the volume of this piece of metal? a. 111.8 ml b. none of these c. 2.706 × 10^3 ml d. 1.118 × 10^5 ml e. 8.945 × 10^–3 ml

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 00:40
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
Asample of an unknown metal (density = 4.920 g/ml) weighs 550.0 g. what is the volume of this piece...
Questions


History, 02.08.2019 18:00



Health, 02.08.2019 18:00

Biology, 02.08.2019 18:00


Physics, 02.08.2019 18:00

Biology, 02.08.2019 18:00

Health, 02.08.2019 18:00








English, 02.08.2019 18:00

Mathematics, 02.08.2019 18:00