
The electronic configuration of an element is given below. 1s22s22p63s1 which statement about the reactivity of the element is true? it is reactive because it has a full outermost energy level. it is unreactive because it has a full outermost energy level. it is reactive because it has to lose one electron to have a full outermost energy level. it is unreactive because it has to gain one electron to have a full outermost energy level.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
The electronic configuration of an element is given below. 1s22s22p63s1 which statement about the re...
Questions




Mathematics, 10.12.2020 19:30

Mathematics, 10.12.2020 19:30


Mathematics, 10.12.2020 19:30


Mathematics, 10.12.2020 19:30


Mathematics, 10.12.2020 19:30

Mathematics, 10.12.2020 19:30


Mathematics, 10.12.2020 19:30



History, 10.12.2020 19:30


Geography, 10.12.2020 19:30

English, 10.12.2020 19:30

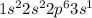 shows that there is only one valence electron present in the outermost shell.
shows that there is only one valence electron present in the outermost shell.

