
Chemistry, 04.08.2019 16:00 NeonPlaySword
One of the reactions for rusting iron is as follows: 4fe + 3o2 → 2fe2o3 (mm fe: 55.85 g/mol; mm o2=32 g/mol; mm fe2o3=159.70 g/mol) if 63.98 g of oxygen gas is completely consumed, how many moles of iron (iii) oxide are formed? a. 1.333 mol b. 3071 mol c. 2.999 mol d. 6812 mol

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
One of the reactions for rusting iron is as follows: 4fe + 3o2 → 2fe2o3 (mm fe: 55.85 g/mol; mm...
Questions



Mathematics, 29.04.2021 23:40


Mathematics, 29.04.2021 23:40

Mathematics, 29.04.2021 23:40


Spanish, 29.04.2021 23:40

Social Studies, 29.04.2021 23:40

Mathematics, 29.04.2021 23:40

English, 29.04.2021 23:40


Mathematics, 29.04.2021 23:40



Computers and Technology, 29.04.2021 23:40

Mathematics, 29.04.2021 23:40

Mathematics, 29.04.2021 23:40

History, 29.04.2021 23:40

English, 29.04.2021 23:40


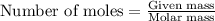
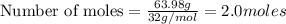
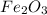 .
.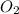 produces 2 moles of
produces 2 moles of 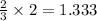 moles of
moles of 

