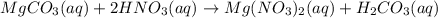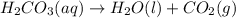Chemistry, 04.08.2019 16:30 aharvitt0417
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, magnesium nitrate and carbonic acid form. carbonic acid then breaks down into water and carbon dioxide. what two types of reactions take place in this process?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, magnesium nitrate and carbonic acid...
Questions

History, 17.04.2020 09:56



Physics, 17.04.2020 09:56



Mathematics, 17.04.2020 09:57

Mathematics, 17.04.2020 09:57

Biology, 17.04.2020 09:57

Health, 17.04.2020 09:57

Mathematics, 17.04.2020 09:57

Health, 17.04.2020 09:57

Chemistry, 17.04.2020 09:57

Biology, 17.04.2020 09:57

History, 17.04.2020 09:57

Mathematics, 17.04.2020 09:57

Mathematics, 17.04.2020 09:57

Mathematics, 17.04.2020 09:57