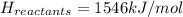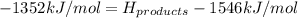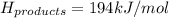Achemical reaction has a change in enthalpy of 1352 kj/mol and a total bonding energy of the recants is 1546 kj/mol. calculate the total bonding energy of the products and decide whether the reaction is endothermic or exothermic a)84 kj/mol, endothermic b)194 kj/mol, endothermic c) 84 kj/mol, exothermic d)194 kj/mol, exothermic

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1


Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Achemical reaction has a change in enthalpy of 1352 kj/mol and a total bonding energy of the recants...
Questions

Mathematics, 12.12.2020 16:10


Biology, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10



Mathematics, 12.12.2020 16:10


Mathematics, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10


History, 12.12.2020 16:10


English, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10



Mathematics, 12.12.2020 16:10

English, 12.12.2020 16:10

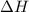 for the reaction comes out to be negative.
for the reaction comes out to be negative.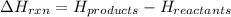
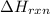 = 1352kJ/mol[/tex]
= 1352kJ/mol[/tex]