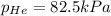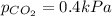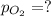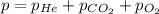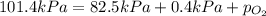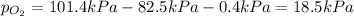Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1
You know the right answer?
Abreathing mixture used by deep-sea divers contains helium, oxygen, and carbon dioxide. what is the...
Questions

Mathematics, 03.12.2021 18:50



Geography, 03.12.2021 18:50



Physics, 03.12.2021 18:50


Mathematics, 03.12.2021 18:50

Biology, 03.12.2021 18:50

Mathematics, 03.12.2021 18:50

Computers and Technology, 03.12.2021 18:50

English, 03.12.2021 18:50




English, 03.12.2021 18:50