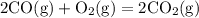Chemistry, 04.08.2019 23:30 jaymariepope3334
In the reaction 2co (g) + o2 (g) → 2co2 (g), what is the ratio of moles of oxygen used to moles of co2 produced?

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

You know the right answer?
In the reaction 2co (g) + o2 (g) → 2co2 (g), what is the ratio of moles of oxygen used to moles of c...
Questions

Mathematics, 01.12.2020 01:00

Computers and Technology, 01.12.2020 01:00









History, 01.12.2020 01:00









English, 01.12.2020 01:00