
Chemistry, 04.08.2019 22:30 kyliemorgan8623
Consider the following three-step representation of a reaction mechanism. step 1: a + b mc016-1.jpg ab (fast) and the rate = k[a][b] step 2: ab + b mc016-2.jpg ab2 (slow) and the rate = k[ab][b] step 3: ab2 + b mc016-3.jpg ab3 (fast) and the rate = k[ab2][b] overall: a + 3b mc016-4.jpg ab3 and the rate = k[a][b]2 which explains why the rate law for the overall equation is not the same as the rate equation for the rate-determining step?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2
You know the right answer?
Consider the following three-step representation of a reaction mechanism. step 1: a + b mc016-1.jpg...
Questions





Mathematics, 16.12.2020 03:50


Mathematics, 16.12.2020 03:50

Arts, 16.12.2020 03:50


Mathematics, 16.12.2020 03:50



Mathematics, 16.12.2020 03:50


Mathematics, 16.12.2020 03:50

Mathematics, 16.12.2020 03:50

Mathematics, 16.12.2020 03:50

Arts, 16.12.2020 03:50


Chemistry, 16.12.2020 03:50

![A+B\rightleftharpoons AB(fast)\\Rate=K[A][B]](/tpl/images/0171/0396/8b588.png)
![AB+B\rightarrow AB_2(slow)\\Rate=K[AB][B]](/tpl/images/0171/0396/fb2ef.png)
![AB_2+B\rightleftharpoons AB_3(fast)\\Rate=K[AB_2][B]](/tpl/images/0171/0396/ac6cb.png)
![Rate=K[A][B]^2](/tpl/images/0171/0396/92d0b.png)
![Rate=K[AB][B]](/tpl/images/0171/0396/058ce.png) ...........(A)
...........(A)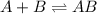
![K=\frac{[AB]}{[A][B]}](/tpl/images/0171/0396/6ec78.png)
![[AB]=K[A][B]](/tpl/images/0171/0396/ad806.png)
![Rate=KK[A][B][B]](/tpl/images/0171/0396/3919b.png)
![Rate=K'[A][B]^2](/tpl/images/0171/0396/8db87.png)


