
Chemistry, 04.08.2019 22:00 connermichaela
Aproposed mechanism for the oxidation of nitric oxide to nitrogen dioxide is shown below. 2 no (g) ⇄ n2o2 (g) fast, reversible step n2o2 (g) + o2 (g) → 2 no2 (g) slow step what rate law is consistent with this mechanism?

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1
You know the right answer?
Aproposed mechanism for the oxidation of nitric oxide to nitrogen dioxide is shown below. 2 no (g) ⇄...
Questions





Chemistry, 19.08.2020 15:01









Mathematics, 19.08.2020 15:01

Mathematics, 19.08.2020 15:01

English, 19.08.2020 15:01


Mathematics, 19.08.2020 15:01


Health, 19.08.2020 15:01

![k_2[N_2O_2][O_2]](/tpl/images/0170/9744/230a2.png)
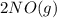 ⇄
⇄ 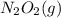 fast, reversible step
fast, reversible step 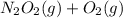 →
→ 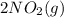
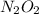 is NOT a reactant or a product. So then we should eliminate it from the rate law.
is NOT a reactant or a product. So then we should eliminate it from the rate law.![k_f [NO]^2 = k_r [N_2O_2]](/tpl/images/0170/9744/b35f7.png)
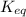 is:
is:
![\frac{k_f}{k_e} = \frac{[N_2O_2]}{[NO]^2} =K_{eq}](/tpl/images/0170/9744/80e33.png)
![[N_2O_2] = \frac{k_f}{k_r} [NO]^2](/tpl/images/0170/9744/c0de9.png)
![[N_2O_2] = \frac{k_f}{k_r}[NO]^2](/tpl/images/0170/9744/dec53.png)
![k_2 [N_2O_2][O_2] = k_2 \frac{k_f}{k_r} [NO]^2[O_2]](/tpl/images/0170/9744/cb3bb.png)
![k_{observed} [NO]^2[O_2] = k_2 K_{eq}[NO]^2[O_2]](/tpl/images/0170/9744/813cc.png)


