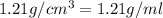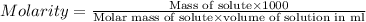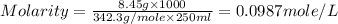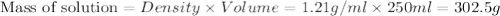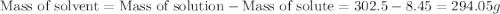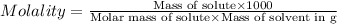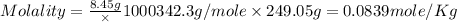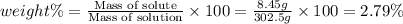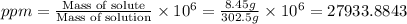Chemistry, 04.08.2019 17:30 fredvales19
Asolution of a sugar with the chemical formula (c12h22o11) is prepared by dissolving 8.45 g in 250.0 ml of water at 25 c. the density of this solution is 1.21 g/cm3. calculate the concentration in terms of molarity, molality, weight percent and ppm. assume that the volume of the solution is equal to the volume of the solvent. show your work.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2
You know the right answer?
Asolution of a sugar with the chemical formula (c12h22o11) is prepared by dissolving 8.45 g in 250.0...
Questions


Spanish, 04.08.2021 06:50

Business, 04.08.2021 06:50


Mathematics, 04.08.2021 06:50

Computers and Technology, 04.08.2021 06:50


Spanish, 04.08.2021 06:50


English, 04.08.2021 06:50


English, 04.08.2021 06:50





Mathematics, 04.08.2021 06:50



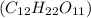 = 8.45 g
= 8.45 g