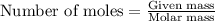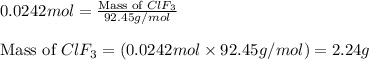Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1


Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
A2.00-l reaction vessel, initially at 298 k, contains chlorine gas at a partial pressure of 337 mmhg...
Questions

History, 17.04.2021 05:10

Mathematics, 17.04.2021 05:10


Mathematics, 17.04.2021 05:10



Mathematics, 17.04.2021 05:10


Mathematics, 17.04.2021 05:10


Biology, 17.04.2021 05:10




Spanish, 17.04.2021 05:10




History, 17.04.2021 05:10

Mathematics, 17.04.2021 05:10

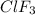 is 2.24 g
is 2.24 g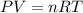 .......(1)
.......(1)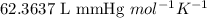
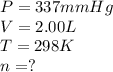
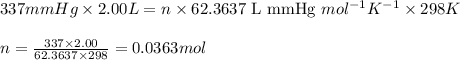
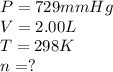
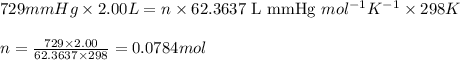
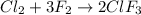
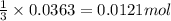 of chlorine gas
of chlorine gas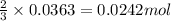 of
of