

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1

Chemistry, 23.06.2019 12:30
Choose one literary selection from this semester in which you think the setting has a great impact on the work. in a full paragraph name the work, describe the setting, and explain why it is so important to the overall story or poem.
Answers: 1

Chemistry, 23.06.2019 13:30
Determine the rate law, including the values of the orders and rate law constant, for the following reaction using the experimental data provided. a + b yields products trial [a] [b] rate 1 0.30 m 0.25 m 1.2 × 10-2 m/min 2 0.30 m 0.50 m 4.8 × 10-2 m/min 3 0.60 m 0.50 m 9.6 × 10-2 m/min
Answers: 1
You know the right answer?
How many molecules are present in 936 g of glucose (c6h12o6)? (the molar mass of glucose = 180.16 g...
Questions


Mathematics, 04.05.2021 17:40

Mathematics, 04.05.2021 17:40

Mathematics, 04.05.2021 17:40

Business, 04.05.2021 17:40

Business, 04.05.2021 17:40

Mathematics, 04.05.2021 17:40

English, 04.05.2021 17:40

Mathematics, 04.05.2021 17:40



Mathematics, 04.05.2021 17:40

Mathematics, 04.05.2021 17:40

Mathematics, 04.05.2021 17:40

Social Studies, 04.05.2021 17:40


Mathematics, 04.05.2021 17:40

English, 04.05.2021 17:40

Biology, 04.05.2021 17:40

Mathematics, 04.05.2021 17:40

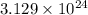
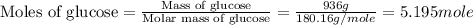
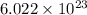 number of molecules.
number of molecules.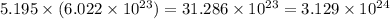 number of molecules.
number of molecules.

