
Chemistry, 03.08.2019 15:30 dezmondpowell
Asolution is prepared by dissolving 20.2 ml of methanol (ch3oh) in 100.0 ml of water at 25oc. the final volume of the solution is 118 ml. the densities of methanol and water at this temperature are 0.782 g/ml and 1.00 g/ml, respectively. for this solution, calculate the molarity, molality, percent by mass, mole fraction, and mole percent.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1
You know the right answer?
Asolution is prepared by dissolving 20.2 ml of methanol (ch3oh) in 100.0 ml of water at 25oc. the fi...
Questions

Mathematics, 21.01.2021 04:10

English, 21.01.2021 04:10

Mathematics, 21.01.2021 04:10



Mathematics, 21.01.2021 04:10

English, 21.01.2021 04:10

Mathematics, 21.01.2021 04:10

Mathematics, 21.01.2021 04:10


History, 21.01.2021 04:10

Mathematics, 21.01.2021 04:10


Chemistry, 21.01.2021 04:10

Social Studies, 21.01.2021 04:10

Social Studies, 21.01.2021 04:10

Biology, 21.01.2021 04:10

Mathematics, 21.01.2021 04:10

Mathematics, 21.01.2021 04:10

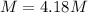
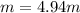
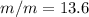 %
%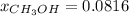
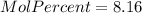 %
%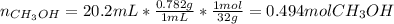
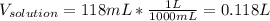
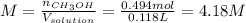
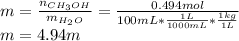
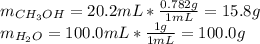
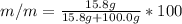 %
%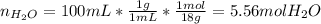
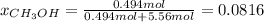
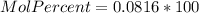 %
%

