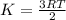What does the kinetic theory state about the relationship between the speed and temperature of gas molecules? as the temperature increases, the speed of gas molecules remains constant. as the temperature increases, the speed of gas molecules decreases. as the temperature increases, the speed of gas molecules increases. as the speed of gas molecules increases, the temperature becomes constant. as the temperature decreases, the speed of gas molecules increases.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1
You know the right answer?
What does the kinetic theory state about the relationship between the speed and temperature of gas m...
Questions

Chemistry, 18.08.2019 15:20

Mathematics, 18.08.2019 15:20


History, 18.08.2019 15:20



Mathematics, 18.08.2019 15:20

Mathematics, 18.08.2019 15:20

History, 18.08.2019 15:20





Mathematics, 18.08.2019 15:20

Mathematics, 18.08.2019 15:20


Social Studies, 18.08.2019 15:20

Chemistry, 18.08.2019 15:20