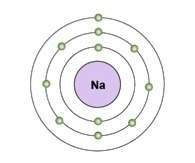
The diagram shows an electron shell model of a sodium atom.
how would the model change a...

The diagram shows an electron shell model of a sodium atom.
how would the model change as the atom forms bonds?
a. the third shell would have eight electrons after the atom gains seven electrons to fill the outermost shell.
b. the third shell would be empty so that the eight electrons in the second level would be outermost after the atom loses one electron.
c. the first and third shells would be empty so that the atom would have eight electrons in its remaining shell after the atom loses three electrons.
d. the first shell would have two electrons and the second shell would have six electrons after the atom loses three electrons.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
You know the right answer?
Questions





Mathematics, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00




Mathematics, 24.02.2021 14:00

English, 24.02.2021 14:00


Chemistry, 24.02.2021 14:00


Health, 24.02.2021 14:00


English, 24.02.2021 14:00

English, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00

French, 24.02.2021 14:00



