
Chemistry, 03.08.2019 05:30 krystlemiller4307
Acombustion analysis of 5.214 g of a compound yields 5.34 g co 2 , 1.09 g h 2 o, and 1.70 g n 2 . if the molar mass of the compound is 129.1 g/mol, what is the chemical formula of the compound?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
Acombustion analysis of 5.214 g of a compound yields 5.34 g co 2 , 1.09 g h 2 o, and 1.70 g n 2 ....
Questions

Social Studies, 27.06.2019 13:30

English, 27.06.2019 13:30

History, 27.06.2019 13:30

History, 27.06.2019 13:30







History, 27.06.2019 13:30




History, 27.06.2019 13:30




Geography, 27.06.2019 13:30

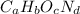 . The mass and molar mass of compound is 5.214 g and 129.1 g/mol respectively.
. The mass and molar mass of compound is 5.214 g and 129.1 g/mol respectively.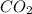 , 1.09 g of
, 1.09 g of 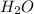 and 1.70 g of
and 1.70 g of 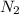 . First number of moles of carbon, hydrogen, oxygen and nitrogen to compare the molar ratio.
. First number of moles of carbon, hydrogen, oxygen and nitrogen to compare the molar ratio.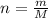
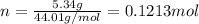
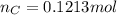
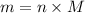
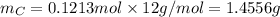
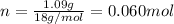
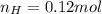
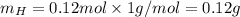
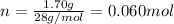
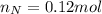
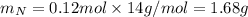
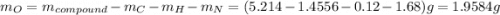
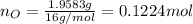
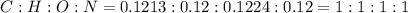
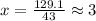
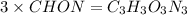
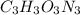 .
.

