
Chemistry, 03.08.2019 03:00 anthonymcnulty6471
The water-gas shift reaction co(g)+h2o(g)⇌co2(g)+h2(g) is used industrially to produce hydrogen. the reaction enthalpy is δh∘=−41kj. to increase the equilibrium yield of hydrogen would you use high or low temperature?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
The water-gas shift reaction co(g)+h2o(g)⇌co2(g)+h2(g) is used industrially to produce hydrogen. the...
Questions


English, 18.11.2020 03:00


Mathematics, 18.11.2020 03:00


English, 18.11.2020 03:00

Mathematics, 18.11.2020 03:00

Biology, 18.11.2020 03:00

Biology, 18.11.2020 03:00

Mathematics, 18.11.2020 03:00

Mathematics, 18.11.2020 03:00

Mathematics, 18.11.2020 03:00

Mathematics, 18.11.2020 03:00

Mathematics, 18.11.2020 03:00



Mathematics, 18.11.2020 03:00

Chemistry, 18.11.2020 03:00

Mathematics, 18.11.2020 03:00

Mathematics, 18.11.2020 03:00

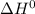 ) for water-gas shift reaction is negative.That means the given reaction is exothermic. So heat is produced in formation of
) for water-gas shift reaction is negative.That means the given reaction is exothermic. So heat is produced in formation of 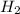 Hence decrease in temperature would lead to shift of equilibrium towards forward direction i.e. formation of
Hence decrease in temperature would lead to shift of equilibrium towards forward direction i.e. formation of  and
and 

