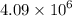Chemistry, 02.08.2019 23:30 jholland03
95 x 1025 molecules of carbon tetrafluoride would have a mass of what?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

You know the right answer?
95 x 1025 molecules of carbon tetrafluoride would have a mass of what?...
Questions






Mathematics, 05.04.2021 16:00

Business, 05.04.2021 16:00

Social Studies, 05.04.2021 16:00

Mathematics, 05.04.2021 16:00


Mathematics, 05.04.2021 16:00

English, 05.04.2021 16:00



Medicine, 05.04.2021 16:00

Mathematics, 05.04.2021 16:00

English, 05.04.2021 16:00

Mathematics, 05.04.2021 16:00

Mathematics, 05.04.2021 16:00