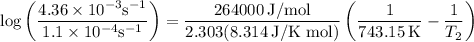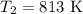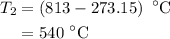Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1
You know the right answer?
The rate constant of a reaction is 1.1 × 10-4 s-1 at 470 °c, and the activation energy is 264 kj/mol...
Questions

World Languages, 02.08.2019 08:00




Computers and Technology, 02.08.2019 08:00

Computers and Technology, 02.08.2019 08:00







Computers and Technology, 02.08.2019 08:00

Computers and Technology, 02.08.2019 08:00

Computers and Technology, 02.08.2019 08:00

Mathematics, 02.08.2019 08:00

Mathematics, 02.08.2019 08:00


Computers and Technology, 02.08.2019 08:00

Computers and Technology, 02.08.2019 08:00

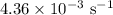 comes out to be
comes out to be  .
.
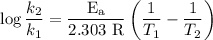 …… (1)
…… (1)
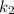 is rate constant at temperature
is rate constant at temperature 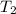 .
.
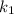 is rate constant temperature
is rate constant temperature 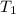 .
.
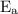 is activation energy.
is activation energy.
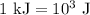
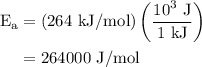
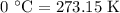
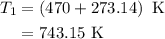
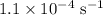 .
.